Updated 6 May 2021 at 06:51 IST
COVID-19 vaccination drive for 18-44 age group halted in Chhattisgarh post HC order
In a big development, the Chhattisgarh government postponed the COVID-19 vaccination of those aged between 18 and 44 based on a High Court order.
- India News
- 3 min read
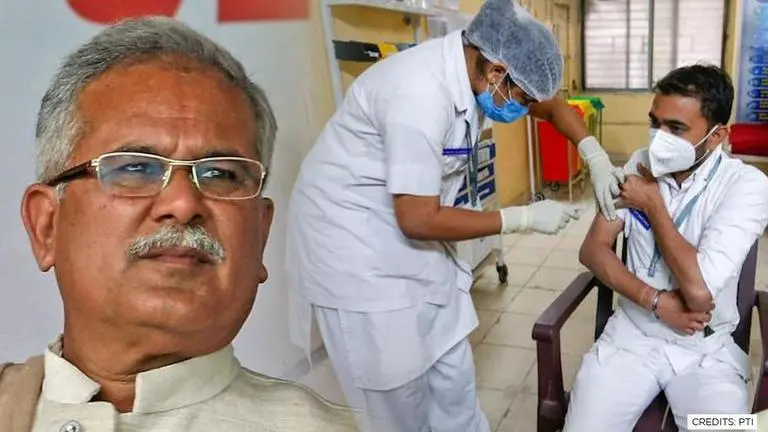
In a big development, the Chhattisgarh government postponed the COVID-19 vaccination of those aged between 18 and 44 based on an HC order. Its decision to inoculate Antyodaya cardholders first followed by those below the poverty line and then those above had been challenged by many petitioners including Janta Congress Chhattisgarh (Jogi) head Amit Jogi. The petitions argued that the state government’s sub-classification is beyond the constitutional mandate and is patently in violation of the law of equality.
Questioning the legality of the state government's plan to tinker with the Centre's vaccination policy, the bench of Chief Justice PR Ramachandra Menon and Justice PP Sahu asked the former to reconsider its stance on prioritising certain socio-economic groups. Maintaining that limited supply of vaccines cannot be a reason to deprive people of their right to access healthcare, it directed the Bhupesh Baghel-led government to take a decision after taking into account aspects such as vulnerability and number of eligible persons in the 18-44 group. Subsequently, the matter was adjourned to May 7.
In a letter addressed to the District Collectors, the state Health Department informed them it cannot go ahead with the current policy as it will be perceived as "contempt of court". Thus, inoculation for all adults is expected to resume only after suitable changes are made to the policy. So far, only 1026 persons in this age group have been administered the vaccine.
Chhattisgarh govt postpones vaccination for the 18 to 44 years age group
— ANI (@ANI) May 5, 2021
“Considering High Court’s directives over the third phase of the COVID-19 vaccination drive, inoculation of the 18 to 44 age group has been postponed until amendments have been done,” the letter reads.
COVID-19 vaccination policy
Apart from COVISHIELD and COVAXIN, the DCGI accepted the recommendations of the Subject Expert Committee of the Central Drugs Standard Control Organisation, paving way for the approval of Sputnik V on April 12. Moreover, the Union government declared that those vaccines that have been granted emergency approval for restricted use by USFDA, EMA, UK MHRA, PMDA Japan, or which are listed in WHO (Emergency Use Listing) will be granted emergency use approval in India. In a huge announcement on April 19, the Centre relaxed the age bar for vaccination from May 1 onwards and mentioned that vaccine manufacturers can supply 50 per cent of its doses to state governments and in the open market.
Advertisement
The private hospitals shall have to procure their supplies of the COVID-19 vaccines exclusively from this quota. At the same time, vaccination shall continue at Government of India vaccination centres for healthcare and frontline workers and those above 45 years. Additionally, the Centre stated that it will allocate vaccine doses to the states from the remaining 50% quota based on factors such as the number of active cases, speed of administration and vaccine wastage.
Published By : Akhil Oka
Published On: 6 May 2021 at 06:51 IST
