Updated 10 April 2020 at 10:45 IST
IMPORTANT: India to deploy Covid-recovered patients' antibodies in plasma therapy trial
India will soon start clinical trials of a plasma treatment for critical COVID-19 patients and Kerala is set to become the first state in the country to start.
- India News
- 3 min read
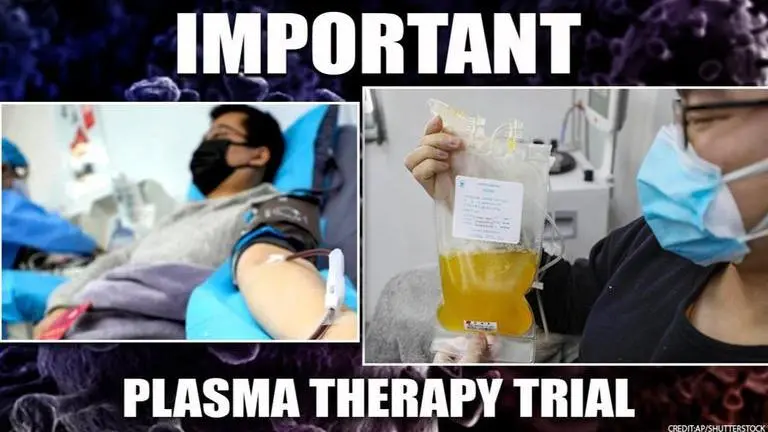
India will soon start clinical trials of a plasma treatment for critical COVID-19 patients, according to the Indian Council of Medical Research (ICMR). Convalescent plasma therapy is a process in which blood plasma from a patient who has recovered from COVID-19 is infused into a critically ill patient so that the specific antibodies present in the blood of the recovered person can help fight the infection.
'A promising rescue option'
Researchers called convalescent plasma therapy a “promising rescue option” for severe COVID-19 patients, but added that large randomised control trials are needed. The study appeared on Tuesday in the American journal, Proceedings of the National Academy of Sciences (PNAS). The trails become important as there is no tried and tested anti-viral drug or vaccine against the novel Coronavirus yet.
According to a PTI report, Kerala is set to become the first state in the country to commence convalescent plasma therapy, which uses antibodies from the blood of cured patients, to treat critically ill COVID-19 cases on a trial basis. The Indian Council of Medical Research (ICMR) has given its nod to the state government for the first of its kind project, initiated by the prestigious Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), a top official said.
'We have received the approval from the ICMR'
SCTIMST, an Institution of National Importance under the Union Department of Science and Technology, is expecting to start the trials by this month-end once the required approvals from the Drugs Controller of India and the Ethics committee are received. "We have received the approval from the Indian Council of Medical Research (ICMR) to conduct it as a clinical trial", Director of the city-based institute Dr Asha Kishore said.
In COVID-19, some small studies have been done in China and the United States where they had tried this treatment method, she said. "We do not have strong evidence that it works. So it will be tested in the form of a clinical trial to see whether it will work or not", the Director said adding they were trying to get Corporate Social Responsibility (CSR) funds for the project, which is estimated to cost Rs 25 lakh.
Advertisement
The project has been approved by the state government and five medical colleges -- at Thiruvananthapuram, Alapuzha, Ernakulam, Thrissur and Kannur -- and an expert form COVID-19 clinic in Kozhikode, Dr Anoop, who will do the clinical follow up, will be participating in it.
Advertisement
Meanwhile, with an increase of 547 new COVID-19 cases in the last 12 hours, India's total number of coronavirus positive cases rose to 6,412 on Friday. Out of the total cases, 5,709 are active patients and 504 of them have been cured/discharged and migrated, as per the Ministry of Health and Family Welfare. With 30 new deaths reported in the last 12 hours, the death toll stands at 199.
(With PTI inputs)
Published By : Jay Pandya
Published On: 10 April 2020 at 10:45 IST
