Updated 24 November 2020 at 21:40 IST
Pfizer, Moderna, Oxford, Sputnik V: As Covid Vaccines publish results, here's what we know
Phase 3 clinical trial data for 4 Covid vaccine candidates has been released in the past few weeks. Here's an analysis of Pfizer, Moderna, Oxford & Sputnik V
- Science News
- 4 min read
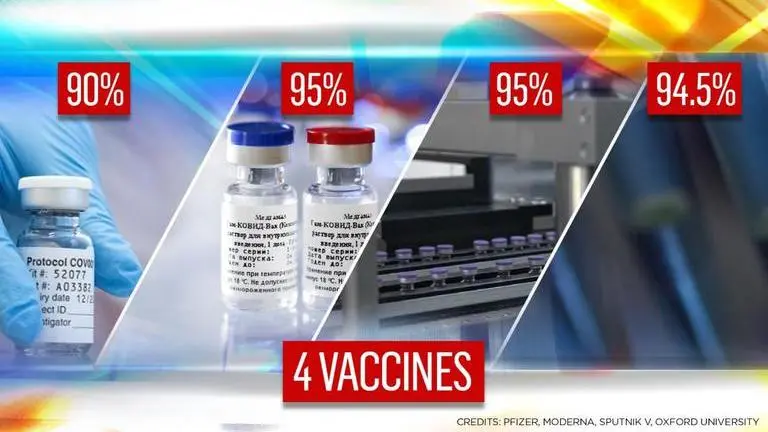
About one year on from what Chinese media records as the first case of COVID-19, it appears that a vaccine that may offer a tangible 'normalcy-restoring' intervention may be within touching distance. In fact, there may be a few such rays of light on hand, which give rise to a flurry of questions that will undoubtedly need answering. But first, here's a download of four of the vaccine candidates that have recently published results from their phase 3 clinical trials:
Pfizer, Moderna, Oxford, Sputnik V: A comparison of Covid vaccines' phase 3 trial efficacy & storage factors
1. Pfizer's Vaccine (also BioNTech, Fosun Pharma) -
Pfizer's vaccine has been demonstrated to be 95% effective against Covid 28 days after the first dose (i.e. 7 days after second dose)
i.e. Dose 1 + 21 days + Dose 2 + 7 days -> 95% efficacy
The results were published after the conclusion of the phase 3 study which enrolled 43,661 participants. It found 170 confirmed cases of COVID-19, with 162 observed in the placebo group (those who didn't get the vaccine) versus 8 in the vaccine group.
Advertisement
Pfizer's storage plan is that it has 'developed specially designed, temperature-controlled thermal shippers utilizing dry ice to maintain temperature conditions of -70°C±10°C. They can be used be as temporary storage units for 15 days by refilling with dry ice.' Essentially, however, the vaccine in its current form needs to be stored at -70°C.
READ: Pfizer's press release/strong>
Advertisement
2. Moderna's Vaccine: (mRNA-1273)
Moderna's vaccine has shown an efficacy of 94.5% two weeks after the second dose in an interim analysis
i.e. Dose 1 + 21 days + Dose 2 + 14 days -> 94.5%
The study enrolled more than 30,000 people, in which 90 cases of Covid were observed in the placebo group versus 5 cases in the vaccine group.
Moderna plans to submit an emergency use authorisation application based on a final study of 151 cases, results of which are expected.
Moderna says storage and handling of the vaccine can be done using existing infrastructure. The vaccine is survivable in a standard medical refrigerator at 2-8 degree Celsius for up to 30 days and longer in a freezer (-4 to -20 degree Celsius)
READ: Moderna's press release
3.Oxford vaccine (University of Oxford, Astrazeneca, Serum Institute):
Interim phase 3 data analysing 131 COVID cases presented two key findings:
The first was that a half dose and then a full dose is 90% effective
i.e. Half Dose 1 + 21 days + Dose 2 + unspecified number of days -> 90% efficacy
The second was that a full dose and then another full dose is 62% effective
i.e. Dose 1 + 21 days + Dose 2 + unspecified number of days -> 62% efficacy
Combining data from the two dosing regimens, the efficacy was found to be 70.4%.
Oxford says, "Crucially, vaccine can be easily administered in existing healthcare systems, stored at ‘fridge temperature’ (2-8 °C) and distributed using existing logistics."
READ: Oxford's press release
4. Russian vaccine Sputnik V:
A second interim analysis of the phase 3 trial data analyses 39 confirmed cases (31 from placebo group, 8 from vaccine group) in a study comprising 18,794 volunteers. It presents two results for the same regimen:
On day 28 after the first dose, a 91.4% efficacy was reported
i.e. Dose 1 + 21 days + Dose 2 + 7 days -> 91.4% efficacy
On day 42 after the first dose, a 95% efficacy was reported
i.e. Dose 1 + 21 days + Dose 2 + 21 days -> 95% efficacy
The vaccine's vials must be stored at -18 degree Celsius or below, though a freeze-dried version is being tested that may be stored in a regular fridge (2-8 degree Celsius).
READ: Sputnik V press release
There are numerous other Covid vaccines in the works, including from India and China, and they are unlikely to want to wait too long to publish their own clinical trial data, interim or final. As per Worldometer, at the time of publishing, almost 60 million Coronavirus cases have been reported worldwide with over 1.4 million people succumbing. There are an estimated 17 million active infections. The vaccination of the world's population is likely to be the single-largest collective undertaking in the history of humanity.
Published By : Ankit Prasad
Published On: 24 November 2020 at 21:20 IST
