Updated 26 January 2021 at 16:13 IST
AstraZeneca trashes reports of only 8% COVID-19 vaccine efficacy in elderly
AstraZeneca said,” In November, we published data in The Lancet demonstrating that older adults showed strong immune responses to the AZD1222 vaccine."
- World News
- 2 min read
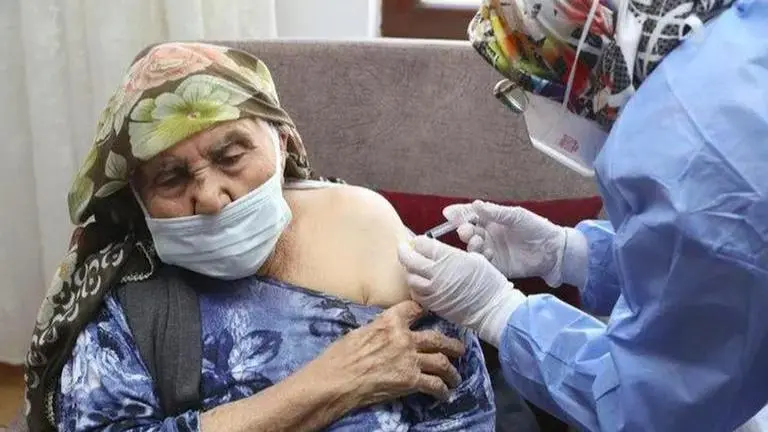
Citing positive high-level results from an interim analysis of clinical trials of its vaccine AZD1222, AstraZeneca on January 25 trashed reports claiming that the pharmaceutical firm is ‘unsure’ about its product’s efficacy among elderly over the age of 65. German daily Handelsblatt reported that the country’s government had calculated Oxford co-manufactured AstraZeneca vaccine to provide only 8 per cent immune response among those that were older than 65. However, in an official statement Monday, AstraZeneca said, “Reports that the AstraZeneca/Oxford vaccine efficacy is as low as 8% in adults over 65 years are completely incorrect.”
Debunking the reports of low efficacy, AstraZeneca said, ”In November, we published data in The Lancet demonstrating that older adults showed strong immune responses to the vaccine, with 100 percent of older adults generating spike-specific antibodies after the second dose.” Following its clinical trials, the pharmaceutical giant had declared its vaccine AZD1222 efficacy to be 90 percent when administered as a half dose, followed by a full dose at least one month apart, and another dosing regimen showed 62 percent efficacy when given as two full doses at least one month apart. The company cited the average efficacy of its vaccine to be 70 percent, adding that an independent Data Safety Monitoring Board determined that the vaccine analysis met its primary endpoint showing protection.
Advertisement
Today at @WEF #DavosAgenda, #DavosAgenda CEO Pascal Soriot emphasised the importance of building resilient healthcare systems to recover from the pandemic through public-private sector collaboration. More on our partnership with WEF and @LSE: https://t.co/1J0NcdVrb8 pic.twitter.com/XboabHe3yI
— AstraZeneca (@AstraZeneca) January 25, 2021
Phone call to AstraZeneca's CEO
Earlier, European Union (EU) lashed out at the pharmaceutical to deliver more coronavirus vaccine doses to its 27 nations bloc, following the critical shortfall of the vials. EU's executive Commission spurred into action as President Ursula von der Leyen's made a phone call to AstraZeneca's CEO Pascal Soriot asking the firm to adhere to the contractual arrangements “foreseen in the advance purchasing agreement.” the delay, according to European press reports, will hamper the EU's planned goals of vaccinating 70 percent of the adult population by June, 2021.
"She reminded Mr Soriot that the EU has invested significant amounts in the company up front precisely to ensure that production is ramped up even before the conditional market authorization is delivered by the European Medicines Agency," Mamer said at a news briefing in Brussels.
We will only defeat #COVID19 when people around the world have access to vaccines. #COVAX is the best route to deliver large quantities of vaccines to lower & middle-income countries. But until COVAX is able to deliver, I propose an EU mechanism to share some of our vaccines. pic.twitter.com/BTJxlF1qI0
— Ursula von der Leyen (@vonderleyen) January 22, 2021
Advertisement
Published By : Zaini Majeed
Published On: 26 January 2021 at 16:15 IST
