Updated 21 June 2020 at 05:02 IST
COVID-19: Cipla, Hetero get DCGI approval to manufacture, sell anti-viral drug Remdesivir
DCGI granted permission to two domestic pharmaceuticals companies, Cipla and Hetero, for sale of anti-viral drug Remdesivir for treatment of COVID-19 patients
- India News
- 2 min read
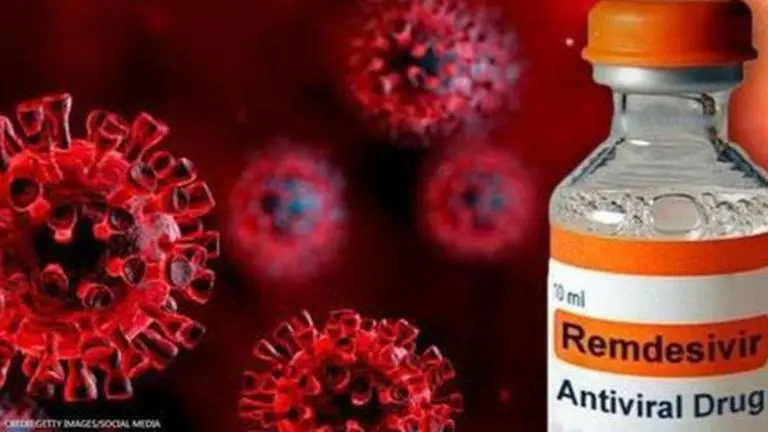
Drugs Controller General of India (DGCI) has granted permission to two domestic pharmaceuticals companies, Cipla and Hetero, for sale of anti-viral drug Remdesivir for treatment of COVID-19 patients with mild symptoms. The top drug regulator gave a nod to the companies on conditions of "restricted emergency use".
With the development, India can now begin the domestic production of anti-viral drugs which would have efficacy, stability, safety for "restricted emergency use" on COVID-19 patients, a senior official told ANI.
Remdesivir is not recommended for patients with severe renal impairment and high level of liver enzymes, pregnant and lactating women, and those below 12 years, Union Health Ministry's document on 'Clinical Management Protocols for COVID-19' stated.
Advertisement
According to reports, the Central Drug Control Standard Organisation (CDCSO) office of DCGI had earlier granted permission to US-based pharmaceuticals giant Gilead Sciences for the marketing of Remdesivir in India for emergency use on patients hospitalised for COVID-19.
Cipla and Hetero Labs have already entered into non-exclusive licensing agreements with Gilead Sciences, which holds the patent for Remdesivir, as per PTI reports.
Advertisement
Emergency use of drugs some allowed
On June 19, India's top drug regulator granted permission to anti-viral drug favipiravir for "restricted emergency use" in mild to moderate cases of COVID-19, a senior government official confirmed to ANI. The Drug Controller General of India (DCGI) has given a fast-tracked permission to Indian drugmaker Glenmark to manufacture and market favipiravir (200 mg) tablet.
The Ministry of Health had on June 14 approved the restricted emergency use of Remdesivir as per the newly updated Clinical Management Protocol for COVID-19. Listed as an investigational therapy along with Convalescent plasma and Tocilizumab, Remdesivir should be used only in a defined subgroup of patients.
(With agency inputs; Image - PTI)
Published By : Sounak Mitra
Published On: 21 June 2020 at 05:02 IST
