Updated 14 October 2024 at 19:26 IST
WHO Approves Bavarian Nordic's Mpox Vaccine For Adolescents
The Jynneos vaccine received prequalification for adolescents on October 8, according to a World Health Organisation (WHO) statement.
- Health News
- 2 min read
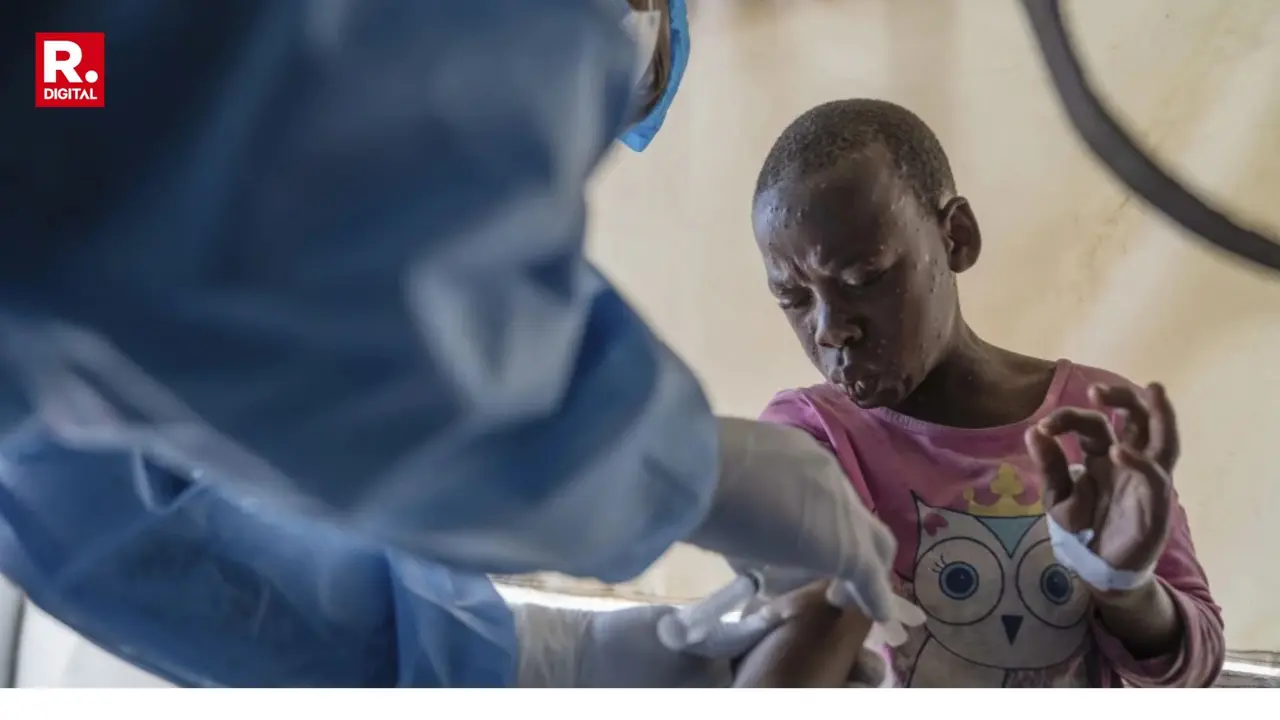
Bavarian Nordic’s Mpox vaccine met with the World Health Organization's approval on Monday for use among adolescents aged between 12-17. This age bracket is considered to be highly vulnerable to Mpox outbreaks that has been deemed as a "public health emergency" by the UN agency responsible for public health.
In an official statement, WHO confirmed that it gave the two-dose Jynneos vaccine prequalification for adolescents on October 8.
The international health agency had approved the use of the vaccine in September after the first shot against mpox in adults, making accessibility simpler for Mpox affected African countries, according to an official release.
Adolescents, and children suffering from weakened immune systems have been particularly vulnerable to mpox, a viral infection that typically causes flu-like symptoms and skin lesions filled with pus, as per media reports.
Advertisement

WHO shows faith in Bavarian Nordic's Mpox shots
WHO’s latest decision comes after the European Union (EU) approved the drug for vaccinating adolescents in September.
The Danish biotech major also plans to conduct clinical trials that test the vaccine's safety in children aged between two-12.
Advertisement
The trial, partially funded by the Coalition for Epidemic Preparedness Innovations, is expected to begin in October, according to media reports.
The U.S. Food and Drug Administration (FDA has also greenlighted Bavarian’s vaccine, however its only for use among 18-year-olds and above. The FDA had 'granted Emergency Use Authorization' for its use in adolescents during the mpox outbreak of 2022.

Mpox vaccine agreement with UNICEF
Earlier, the vaccine developing firm had also inked an agreement with UNICEF to supply 1 million doses of the MVA-BN mpox vaccine for Africa nations impacted by the Mpox outbreak.
In September, Paul Chaplin, President & CEO of Bavarian Nordic, had said," This agreement has helped to secure more than 2.5 million doses of MVA-BN, thus fulfilling the short-term requirement as expressed by the Africa CDC," citing a company release.
During the same period, Bavarian Nordic was also awarded an order worth USD 63 million to manufacture additional bulk product and final freeze-dried doses of JYNNEOS smallpox/mpox vaccine.
Published By : Nitin Waghela
Published On: 14 October 2024 at 19:23 IST
