Updated 27 February 2021 at 20:37 IST
Centre caps vaccine doses at Rs 250 at pvt hospitals; free of charge at govt centres
Union Health Secretary Rajesh Bhushan on Saturday, announced that pricing for a vaccine dose has been capped at Rs 250 at private hospitals, free at govt hosp
- India News
- 2 min read
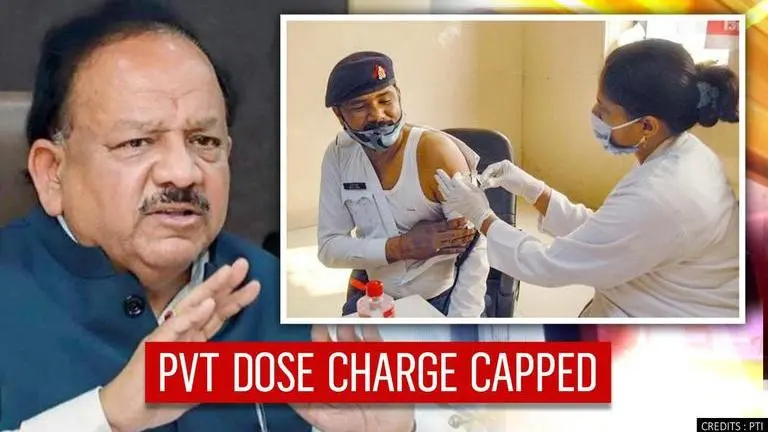
As India readies for phase-2 of COVID-19 vaccination, Union Health Secretary Rajesh Bhushan on Saturday, announced that pricing for a vaccine dose has been capped at Rs 250 at private hospitals. While vaccine doses will be available free of cost at government centres, Centre will bear the cost of the inoculation. As of date, 77% of health care workers have been administered with the first dose and 70% got the second dose - totalling 1,35,60,983 doses.
Vaccine doses capped at Rs 250/dose at pvt hospitals
"Private hospitals functioning as COVID-19 Vaccination Centres may recover a charge subject to a ceiling of Rs 250 per person per dose. To ramp up COVID vaccination capacity a large number of private facilities are being involved- around 10,000 private hospitals empanelled under Ayushman Bharat PMJAY, more than 600 hospitals empanelled under CGHS & other private hospitals empanelled under State Govts," said Bhushan. Phase-2 of vaccination for people above 60 years of age and those aged above 45 years having comorbidities will begin on March 1.
India's vaccine drive
On January 3, Drug Controller General of India (DCGI) VG Somani announced that the vaccines of Serum Institue of India (Covishield) and Bharat Biotech (Covaxin) have been granted permission for restricted use in an emergency situation. Covishield - a Recombinant Chimpanzee Adenovirus vector vaccine - has an efficacy of 70.42%, with interim safety and immunogenicity data of Phase-II/III trials submitted to the SEC. Covaxin - a Whole Virion Inactivated Corona Virus Vaccine - 22,500 participants vaccinated in Phase-III trials and was found to be safe as per the data available till date. Meanwhile, Zydus-Cadilla's nCov-Vaccine using DNA platform technology has been allowed to conduct Phase-III clinical trial in 26000 Indian participants. Apart from these vaccines, another vaccine candidate - Sputnik V - produced by Russian government-Dr. Reddy's Labs has applied for emergency use and Bharat Biotech has sought to conduct phase-1 trials of nasal COVID-19 vaccine.
Advertisement
With Recovery Rate improving to 97.14%, India has become the second-fastest fastest to administer 10 million COVID-vaccines doses - in 34 days. In comparison, the US - the fastest - took 31 days, stated the Ministry of Health and Family welfare. The UK and the US, both kick-started their inoculation drives in December.
#LargestVaccineDrive
— Ministry of Health (@MoHFW_INDIA) February 19, 2021
India has attained the third position globally in terms of Total Vaccination administered with over 10.1 million vaccine doses against #COVID19. pic.twitter.com/h8inctPeRg
Advertisement
Published By : Suchitra Karthikeyan
Published On: 27 February 2021 at 20:37 IST
