Updated 3 March 2021 at 17:45 IST
Bharat Biotech's Covaxin shows 81% efficacy in Phase 3 trials conducted on 25k subjects
Covaxin's phase 3 trials involved 25,800 subjects and is the largest ever trial conducted in India in collaboration with ICMR, Bharat Biotech said on Tuesday.
- India News
- 3 min read
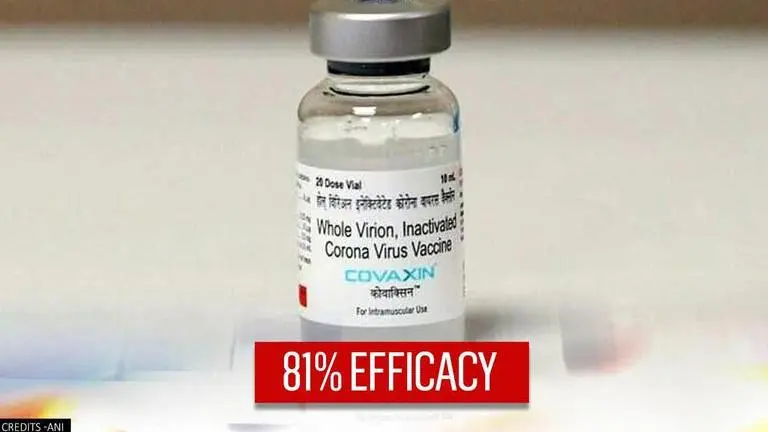
Two months after being granted permission for restricted use in India, Hyderabad-based pharmaceutical firm Bharat Biotech has said that its COVID-19 vaccine - Covaxin - has shown an interim clinical efficacy of 81% in its Phase 3 trials. The phase 3 trials involved 25,800 subjects and is the largest ever trial conducted in India, Bharat Biotech said on Tuesday. The vaccine has been in use since India began its first phase of the COVID-19 immunization drive back in January this year.
Elaborating on the details of the Phase 3 study, the pharma firm said that the participants enrolled were between the age of 18-98 years old including 2,433 over the age of 60 and 4,500 with comorbidities. "The primary endpoint of Phase 3 clinical trial is based on the first occurrence of PCR-confirmed symptomatic (mild, moderate, or severe) COVID-19 with onset at least 14 days after the second study vaccination in serologically negative (to SARS-CoV-2) adult participants at baseline," Bharat Biotech added.
COVAXIN has demonstrated an interim vaccine efficacy of 81% in its Phase 3 clinical trial. The trials involved 25,800 subjects, the largest ever conducted in India, in partnership with ICMR: Bharat Biotech pic.twitter.com/jDIka9LIEE
— ANI (@ANI) March 3, 2021
"Today is an important milestone in vaccine discovery, for science and our fight against coronavirus. With today's results from our Phase 3 clinical trials, we have now reported data on our COVID-19 vaccine from Phase 1, 2, and 3 trials involving around 27,000 participants," Bharat Biotech Chairman and Managing Director Krishna Ella said.
Bharat Biotech's Covaxin has been indigenously developed in collaboration with the Indian Council of Medical Research (ICMR). It is one of the two COVID vaccines cleared by the DCGI for emergency use in India. The other vaccine - Covishield, manufactured by Serum Institute - has reported 70% efficacy after administration of the second dose. The nod given to Covaxin by the government even before the results of phase 3 trials had invited sharp criticism. Meanwhile, the Made in India vaccine has also begun exporting doses to several countries under India's 'Vaccine Maitri' initiative.
Advertisement
The interim results of the clinical trials had earlier indicated that the Indian COVID-19 vaccines would be effective against the mutated virus strains reported from the United Kingdom, South Africa, and Brazil said the Indian Council of Medical Research (ICMR).
Advertisement
PM Modi takes Covaxin jab
As India began its second phase of inoculation from Monday, Prime Minister Narendra Modi received his first dose of the Covaxin at the AIIMS hospital in the national capital. PM Modi tweeted a picture of himself receiving the COVID vaccine and lauded the doctors and scientists for spearheading the global fight against COVID-19. Furthermore, he also appealed the eligible people to take the vaccine, when made available.
As the Prime Minister received the Covaxin jab, Bharat Biotech said that it will give a ‘huge boost’ to the nation’s immunization campaign and further reduce the hesitancy among the citizens. Dr. Krishna Ella, Chairman & Managing Director, Bharat Biotech International Limited also expressed gratitude to the Prime Minister for setting a “powerful example”. Ella said, “We thank the Hon’ble Prime Minister Sh., Narendra Modi, for taking the first dose of the COVID-19 vaccine himself and are deeply appreciative of the gesture.”
The second phase of the COVID-19 vaccination program started at over 10,000 designated government facilities and 20,000 private hospitals and vaccination centres on Monday. While the first phase of the Coronavirus vaccination drive focused on healthcare and frontline workers, the second phase is dedicated to those above 60 years of age and those above 45 years with co-morbidities.
Published By : Jitesh Vachhatani
Published On: 3 March 2021 at 17:33 IST
